



Artículos originales
Microelements in seeds of advanced genotypes of Caupí beans (Vigna unguiculata L. Walp) established in a soil of Cordoba, Monteria.
Microelementos en semillas de genotipos avanzados de frijol caupí (Vigna unguiculata L. Walp) establecidas en un suelo de Córdoba, Montería.
Temas Agrarios
Universidad de Córdoba, Colombia
ISSN: 0122-7610
ISSN-e: 2389-9182
Periodicity: Semestral
vol. 27, no. 2, 2022
Received: 08 June 2022
Accepted: 10 June 2022

Abstract: The objective was to quantify the nutritional contents in seeds of different advanced genotypes of cowpea bean (Vigna unguiculata L. Walp) in the soil of Montería Córdoba, Colombia. Seeds of 40 lines or genotypes and two commercial varieties Caupicor 50 and ICA Betancí were evaluated as controls. A completely randomized block design was used with three repetitions for a total of 126 experimental units. The seeds were initially subjected to drying in a forced circulation oven at 70°C for 72 hours to quantify the nutritional content. The final nitrogen content was evaluated by initially submitting the seeds to digestion in concentrated sulfuric acid for 4 hours. It was subsequently quantified using the Kjeldahl method. Finally, to determine the content of microelements, 1 g of seed was subjected to digestion with nitric, perchloric acid (3:1), and they were quantified in a piece of atomic adsorption equipment. With the information recorded in the laboratory, an analysis of variance, contrasts, and tests of Tukey averages was performed at a 5% probability. The results indicate that it existed genetic variability and genotypes LC-041-016 and LC021016 were identified with 29.2 and 29.1%, with the highest protein content, being superior to Caupicor 50 and ICA Betancí that presented contents of 25, 7 and 25.5%. The genotypes L-047 with 216.3, followed by genotypes LCPM35 and LC027016, with 159.5 and 127.3 mg.kg-1 presented the highest iron content, and the commercial witnesses Caupicor 50 and ICA Betancí, content less than the above, and the content zinc and manganese were similar in all genotypes evaluated.
Keywords: Iron, Zinc, Copper, Manganese, Minerals, Biofortification.
Resumen: El objetivo fue cuantificar los contenidos nutricionales en semillas de diferentes genotipos avanzados de frijol caupí (Vigna unguiculata L. Walp) en un suelo de Montería, Córdoba, Colombia. Se evaluaron las semillas de 40 genotipos y dos variedades comerciales Caupicor 50 e ICA Betancí como controles. Se utilizó un diseño de bloques completamente al azar con tres (3) repeticiones. Para cuantificar el contenido nutricional, las semillas fueron sometidas inicialmente a secado en horno de circulación forzada a 70 ° C durante 72 horas. El contenido de nitrógeno se evaluó sometiendo inicialmente las semillas a una digestión en ácido sulfúrico concentrado durante 4 horas. Y posteriormente se cuantificó por el método del método Kjeldahl. Finalmente, para determinar el contenido de microelementos, 1 g de semilla se sometió a digestión con ácido perclórico nítrico (3: 1), y se cuantificaron en un equipo de adsorción atómica. Con los datos un análisis de varianza, contrastes y las pruebas de los promedios de tukey se realizaron con una probabilidad del 5%. Los resultados indican que existía variabilidad genética y los genotipos LC-041-016 y LC021016 se identificaron con 29.2 y 29.1%, con el mayor contenido de proteína, siendo superior a Caupicor 50 e ICA Betancí que presentaron contenidos del 25, 7 y 25.5%. Los genotipos L-047 con 216.3, seguidos por LCPM35 y LC027016, con 159.5 y 127.3 mg.kg-1 presentaron el mayor contenido de hierro, que los testigos comerciales Caupicor 50 e ICA Betancí, y los contenidos de zinc y manganeso fueron similares en todos los genotipos evaluados.
Palabras clave: Hierro, Zinc, Cobre, Manganeso, Minerales, Biofortificación.
INTRODUCTION
In Colombia, there are problems related to food insecurity of several population groups, especially the poorest and most vulnerable that are located in rural areas and urban peripheries. Groups of people that are located within the poverty and extreme poverty indicators of the country, corresponding to values of 31 and 9.1% respectively (FAO, 2013).
These quantities are exceeded in the Caribbean region, mainly in the departments of Guajira and Córdoba with values of 55.8 and 51.8% for poverty and 25.7 and 18.6% for extreme poverty, with a quantity that is above 12%. Therefore, there is chronic malnutrition that affects the future of new generations, as indicated by high rates of infant mortality with a value of 21.6% representing a significant social gap (DNP, 2014).
According to White et al. (2016) and Graham et al. (2007) on the planet, 60% of six billion inhabitants recorded deficiencies in iron (Fe), and 30% in zinc (Zn). In addition, copper (Cu) is deficient in developed and underdeveloped countries, which is attributed to the low availability of mineral elements in the soil and the low capacity of plants to store them in their tissues, associated at the same time with the scarce availability of food of animal origin. According to Bouis et al. (2003) nutrient-deficient eating habits cause vision problems, cognitive development disorders, physical growth, serious episodes of illnesses and high mortality, which has been called "hidden hunger".
Therefore, world agriculture presents a new approach, in which it is sought to improve the nutritional content of species associated with food security, to reduce the problems of malnutrition due to mineral deficiencies in eating habits. Consequently, crop biofortification provides a way to reach populations that are undernourished in relatively remote rural areas, which leads to improved access to fortified foods for these populations (Nestel et al., 2006). Thus, Masuda et al. (2008) have faced this situation as a universal strategy of fortification of food, supplementation and diversification of the diet.
Currently, in Córdoba-Colombia, the genetic improvement of the bean crop seeks a complex interaction between new lines or varieties with high yields, associated with a high nutritional quality of grains, and that these present high protein and microelement contents (Magulu and Kabambe, 2015). According to Araméndiz et al. (2016) in 2013, Araméndiz et al. (2011), report that 11,926,786 hectares were harvested in the world with an average yield of 521 kg ha-1 (FAO, 2013), while in Colombia the cultivated area was 14.000 hectares with an average yield of 600 kg ha-1, especially in the Colombian Caribbean in small plots of farmers who have between 1,000 and 10,000 m2 to farm, and some genotypic lines present good agronomic behvior and grain yield, that can be subjected to evaluation in different environments to determine their phenotypic stability.
Vargas et al. (2012), the cowpea bean (Vigna unguiculata L. Walp.) is a species native to Africa of high resistance and adaptability to different altitudes and types of soils. It is widely cultivated in tropical countries of Africa, South America, Asia, and the southern United States, where this legume has been studied for its nutritional properties. Gupta et al. (2010) found protein percentages in studies conducted on cowpea bean seeds that ranged between 21.2 and 27.9% iron 6.8 mg 100 g–1, manganese 4.1 mg 100 g–1, and phosphorus 1.5 mg 100 g–1. Antova et al. (2014) reported values between 20.1 and 25.8% protein.
Araméndiz et al. (2016) found zinc contents that ranged between 43.45 ± 2.37 mg kg-1 on the C002 line and, 53.38 ± 4.84 mg kg-1 on the L-047 line. Rub et al. (2008) found in beans 24.5 g100 g–1 of protein and among the main iron, zinc and manganese 6.8 microelements: 4.1 and 1.5 mg 100 g–1. However, Salgado et al. (2005) explain that chemical determinations of bean grains must continue, in order to identify and select new superior genotypes, seeking to improve the chemical composition of the grain. Therefore, the objective was to quantify the nutritional contents in seeds of different advanced genotypes of cowpea bean (Vigna unguiculata L. Walp) in the soil of Montería-Córdoba, Colombia.
MATERIALS AND METHODS
Location
This research was carried out in 2017 in the experimental area of the University of Córdoba, located at 8° 45' North latitude and 75° 53' West longitude with an elevation of 15 meters above sea level, in Monteria, Córdoba, Colombia. The annual rainfall is 1346 mm, the average temperature of 27.4 degrees, 2108 hours of annual solar brightness and relative humidity of 85% (Palencia et al., 2006).
In the soil, where the experiment was established a soil sample was collected at a depth of 0 to 20 cm. Later it was taken to the soil laboratory of the Faculty of Agricultural Sciences for the analysis of physical and chemical characteristics according to the methodology of the I.G.A.C (2006).
Table 1 shows that the soil pH showed a slightly acidic reaction (6.44), medium organic matter content (1.58%) and medium sulfur (18 mg kg-1). The contents of nutritional elements such as phosphorus (P) were low, calcium (Ca) and magnesium (Mg) had high contents and medium potassium (K). As for the minor elements, low copper (Cu), iron (Fe) and boron (B) contents were found, the zinc (Zn) contents were medium and manganese (Mn) high. In general, these chemical conditions are indicators of medium fertility.
| Units | |||
| Characteristics | Values | ||
| Unit | Interpretation | ||
| pH | 6.44 | ----------- | Acid |
| M.O | 1.58 | % | Low |
| P | 9.5 | Low | |
| mg kg-1 | |||
| S | 18 | half | |
| Ca | 11.2 | high | |
| Mg | 7.4 | high | |
| K | 0.24 | half | |
| Na | 0.07 | cmolc kg-1 | Low |
| CICe | 18.9 | half | |
| Cu | 0.2 | Low | |
| Zn | 2.4 | half | |
| Fe | 1.8 | Low | |
| Mn | 34.5 | mg kg-1 | high |
| B | 0.2 | Low | |
| Arena | 9.2 | ||
| Arcilla | 36.1 | % | Franco arcillo limoso |
| Limo | 54.8 | ||
Biological material
The population under investigation corresponded to advanced lines developed by the genetic improvement program of the University de Córdoba and conserved in the cowpea bean germplasm collection in the plant breeding laboratory of the University de Córdoba. Seeds of 40 lines or genotypes and two commercial varieties Caupicor 50 and ICA Betancí were evaluated as controls.
Nutritional analysis
The seeds were initially subjected to drying in a forced circulation oven to quantify their nutritional content (Binder - Kasai, Tuttlingen, Germany) at 70 ° C for 72 hours. Next, the seeds of each genotype were ground and passed through a 1 mm sieve to obtain smaller fractions, and then stored in hermetically sealed plastic containers.
To determine the total nitrogen content, 0.5 grams of each material was weighed, which were subjected to digestion in concentrated sulfuric acid for 4 hours, subsequently distilled and collected in 2% boric acid, and titrated with sulfuric acid to 0.25 normal, by the Kjeldahl method, in a Buchi K-355 equipment (Flawil - Switzerland). Finally, to obtain the protein content the nitrogen was multiplied by the factor 6.25.
The content of microelements was determined from a sample of 1 g of each genotype, initially subjected to digestion with nitric perchloric acid (3:1) for one day for greater ion dissolution. Subsequently, the sample was subjected to preheating in an oven to remove plant tissue that was not initially destroyed, obtaining a homogeneous solution. Finally, the microelements were quantified in a Perkin Elmer 3110 atomic adsorption equipment (Norwalk, Connecticut, USA), according to the IGAC methodology (2006).
Analysis of data
A completely randomized block design was used with 42 treatments including the two controls (caupicor 50 and Ica Betancí) and three (3) repetitions, with a total of 126 experimental units. With the information recorded in the laboratory, an analysis of variance, contrasts and tests of tukey averages was performed at 5% probability.
RESULTS AND DISCUSSION.
The average contents of the nutritional elements showed highly significant differences (p≤0.01), except manganese, which were statistically similar for all cultivars (Table 2). These results have been explained by the preponderant genetic dissimilarity among the cultivars studied. Likewise, the pressure of the edaphic- environmental factors influenced in a similar way so that this response will be presented. Carvalho et al. (2012) in cowpea bean, Espinoza García et al. (2016) in common beans and Nair et al. (2015) in mung beans explain the probable existence of a broad genetic structure among these crops, that affect the different concentrations of these nutritional chemical elements.
Similarly, Table 2 shows that when statistically performing the contrasts between the Caupicor 50 and ICA betancí witnesses with the advanced genotypes, only significant differences were presented for the protein and iron variables. This result shows that for these varieties, the genes that determine these characters are similar to at least one of the lines, most likely in their size and position.
Results that indicate that the crops have a differential accumulation in the protein and mineral content in the seed and allows the identification of genotypes of better nutritional quality, for use as food enriched in these nutrients. Feil et al. (2005) indicate that identifying germplasm lines with improved grain yield and quality, especially with respect to mineral content, is an important task in the context of malnutrition caused by the lack of mineral nutrients in underdeveloped countries.
| Source of variation | Gl | protein | Fe | Zn | Cu | Mn |
| Treatments | 41 | 10,45** | 3181,24 ** | 27,85** | 11,45** | 6,32 ns |
| Caupicor vs genotypes | 1 | 0,27 ns | 0,23ns | 0,40 ns | 0,09 ns | 0,21 ns |
| ICA betanci vs genotypes | 1 | 0,0020*** | 0,04** | 0,33ns | 0,38 ns | 0,62 ns |
| Error | 84 | 0,98 | 94,74 | 9,6 | 2,67 | 3,74 |
| C.V (%) | - | 3,76 | 15,32 | 7,18 | 21,27 | 13,65 |
| R2 | - | 0,84 | 0,94 | 0,59 | 0,68 | 0,45 |
Protein
The highest protein contents were found in genotypes LC-041-016 and LC021016 with 29.2 and 29.1%, while the Caupicor 50 and ICA Betancí controls showed lower values with 25.67 and 25.53% respectively (Figure 1). These results are within the range estimated in 43 genotypes of V. unguiculata by De Paula et al. (2018), and they are similar and superior to those documented by Antova et al. (2014) with 25.6% and Aramendiz et al. (2016) with 26.9%. Kouchi et al. (2010) analyze that the genetic study of nodulation offers an understanding of nitrogen fixation symbiosis, and the amount of nodules that are formed in a symbiotic interaction is due to the spatial location of the nodules (Ferguson et al., 2010).
Martínez y Martínez, (2006) explains that proteins are macromolecules formed by chains of amino acids, the main structural and functional component of cells, and fulfill numerous functions within the body. Therefore, the presence of protein in the diet is important because it provides amino acids for the subsequent maintenance of body protein, the most important characteristic being that they contain nitrogen.

The genotypes that presented the lowest protein content in grains were: LC042016, LC026016, L047 and LC025016 with 23.8;23.2; 23.1 and 22.6%. Results that allow the identification of genotypes of lower nutritional quality for use as food and of genotypes that can be excluded from genetic improvement programs. On the other hand, we must bear in mind that these responses are also due to the genetic constitution, the environment where they were established, and the agronomic management to which they were subjected. According to Sadras et al. (2013), the prediction of the phenotypic response based on genetic and environmental factors is the basis for decisions that involve the selection of genotypes with higher nutritional quality for a defined environmental range.
Iron
The highest iron contents were found for genotypes L-047 with 216.3, followed by LCPM35 and LC027016, with 159.5 and 127.3 mg kg-1 which presented significant differences (p ≤ 0.05) with respect to the other genotypes (Figure 2). Values that in general were like those reported by Mesquita et al. (2007) with 126.9 mg kg-1 in 21 bean lines and Vargas et al. (2012) with 138 mg kg-1. On the other hand, in the control lines, Caupicor, 50 showed values higher than 65.17 mg kg-1) and ICA Betancí with 70,230 mg kg-1. Which shows that there are genotypes that can store a higher concentration of iron than Caupicor, 50 and ICA Betancí.
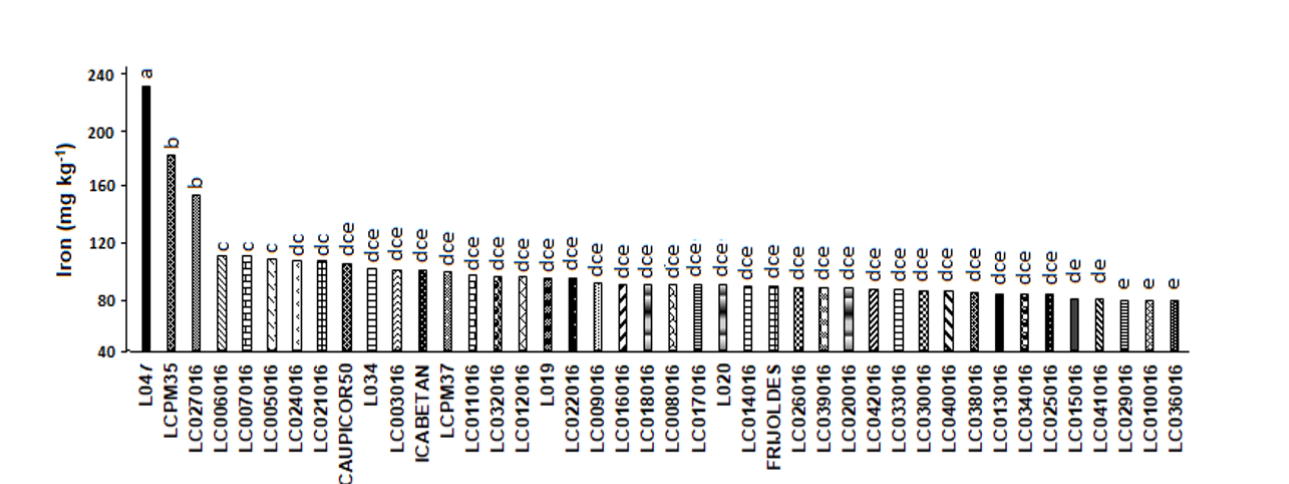
The genotypes with the lowest iron content in seeds were LC029016, LC010016 and LC036016 with 40.4, 40.4 and 40.08 mg kg-1. Contents smaller than those found by Aramendiz et al. (2016) who found values between 59.54 in genotype L-037 and 177.9 mg kg-1 in L-042, and in an original Creole- Cordoba population, recorded values of 60.99 mg kg-1. Similar contents were reported by Ramos and Sangronis (2006) with 98 mg.kg-1 in two bean varieties.
Under these soil conditions, iron contents were deficient, but in the seed high contents were found in some genotypes. Explained by greater physiological efficiency, where there were factors controlled by genes, such as root growth. Which is decisive for iron absorption, especially the young lateral roots that are more active. On the other hand, there are mechanisms for releasing reducing compounds by the roots to lower the pH and chelation process that were manifested frequently between the lines (Saletta et al., 2010). According to Aguado et al. (2012) This chelation process consists of the solubilization of edaphic iron through exudates, caused by the siderophores that are determined by genes.
The intake of this element can supply the amount of iron needed. Therefore, the search for materials rich in this element is important to meet human needs, especially in regions with high indicators of anemia (Aramendiz et al., 2016). This nutritional deficiency is the most widespread in the world, it is estimated that approximately one third of the world population (32.9%) suffered from anemia in 2010 (Kassebaum et al., 2014), and is associated with increased morbidity and mortality in women and children. Furthermore, the decreased labor productivity in adults and impaired cognitive and behavioral development in children (Black et al., 2013; Scott et al., 2014).
Zinc
In the bean genotype seeds, high zinc contents were found, without statistical differences with the controls, the genotypes LC-027-016, L047 and L034 being 51.5; 48.8 and 48.1 mg kg-1 were those who presented the largest contents. Likewise, the ICA Betancí and Caupicor 50 witnesses presented values of 44.8 and 44.6 mg kg-1, but lower than the previous genotypes (Figure 3). These concentrations were higher than those reported by Astudillo and Blair (2008) with 30.73 mg kg-1 in beans and Tolga et al. (2018) with 28.4 mg kg-1, but lower than those reported by Aramendiz et al. (2016) with 53.38 mg kg-1 in beans.
These differential contents are associated with the genotype-environment interaction. As explained above, being that in the soil acceptable zinc contents were presented and in some genotypes, the contents were significantly high. This is associated with greater essentiality of the element in the metabolism and consequently a greater demand, which manifested itself in a differential absorption between genotypes, as reported by Carvalho et al. (2012).

The genotypes with the lowest zinc content in the seeds were LC041016 and LC008016, with 38.7 and 37.3 mg kg-1. Contents smaller than those found Aramendiz et al. (2016) that oscillated between 43.45 and 53.38 mg kg-1 in genotypes C002 and L-047. Iron and zinc deficiencies result in weak growth of children, reduced immunity, weakness, and morbidity (Stein, 2010). According to Duc et al. (2010) producing micronutrient-enriched (biofortified) cultivars, particularly those with agronomically or genetically increased Zn and Fe and improving the bioavailability of these nutrients are considered promising and cost-effective methods to manage micronutrient deficiencies.
Copper
The highest copper contents were found for genotypes L-047 with 12,393 mg kg-1, followed by genotypes LC026016 and LCPM37 with 11.3 and 11.7 mg kg-1 which did not show significant differences (p≤ 0.05) among them, as well as with other genotypes (Figure 4). These contents were lower than Mesquita et al. (2007) with 17.73 mg kg-1.

In relation to the witnesses, Caupicor 50 presented values of 9.3 mgkg-1 and ICA Betancí of 7.803 mgkg-1, without statistical differences between them and with the other genotypes (p ≤ 0.05). These contents were similar to the estimates of Belane and Dakora (2011), which were 8 mg kg-1, and Tolga et al. (2018) with 8.13 mg kg-1.
The lowest copper contents were estimated for genotypes LC-014-016 and LC039016 with 4.7 and 5.2 mg kg-1. Contents lower than the witnesses Caupicor 50 and ICA Betancí and with the other genotypes evaluated. According to Reilly (2004), copper has structural functions and is a cofactor of catalytic activity. It is involved in a large number of biological processes, from immune and neuronal functions to bone and blood health, or antioxidant defense. Similarly, Cu deficiency is associated with alterations in cholesterol metabolism, suggesting that a food intake with a high Cu content is associated with a better metabolic profile (Bost et al., 2016).
In this study, soil copper was deficient, but in the seeds, differential contents were found, suggesting that genotypes did not assimilate the same amount. A response that is due to the chemical variables of the soil (such as the content of organic matter and a pH less than 6, are edaphic conditions that predispose soluble contents of this element. This indicator highlights the importance of the genetic factor, which together with the edaphics explain why some genotypes presented higher contents than others. According to Naz et al. (2013), the distribution and diversity of plants in a specific plant community can be influenced by a variety of factors, such as climate, topography, and soil conditions.
Manganese
The highest manganese contents were found for the LC-002-016 line with L034 17.2 mg kg-1. In the witnesses, Caupicor 50 and ICA Betancí presented values of 15.6 and 16.3 (p ≤ 0.05), without statistical differences between them (Figure 5). These contents were lower than Mesquita et al. (2007) with 17.73 mg kg-1.
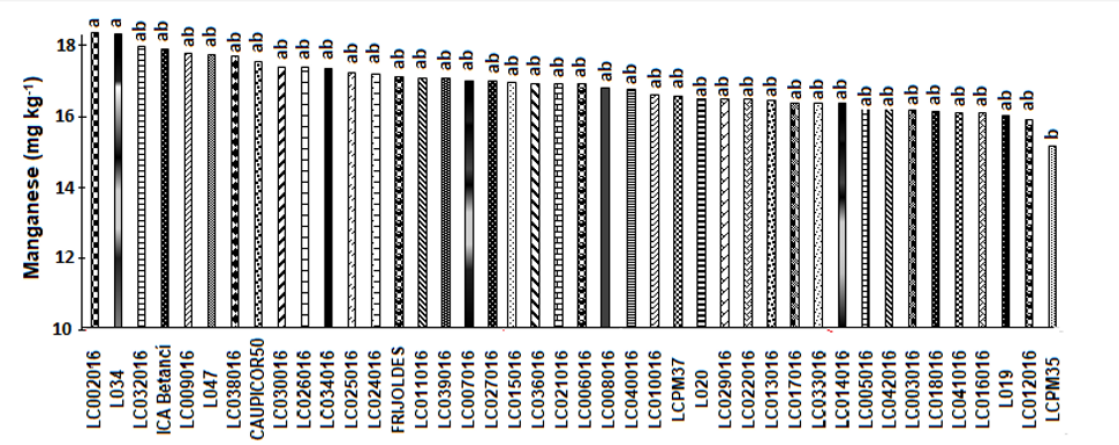
Studies conducted by Belane and Dakora (2011) and Mesquita et al. (2007) found superior results of 40.1 mg kg-1 and 28.9 mg kg-1. However, they were superior to those found by Tolga et al. (2018) who reported values of 24 mg kg-1 and Frota et al. (2008) with 15 mg kg-1. The lowest contents were 10.5 and 12.1 in the LCPM35 and LC012016 genotypes. According to Li (2018) manganese is essential for bone formation and for the metabolism of amino acids, lipids, proteins and carbohydrates. This trace element is necessary for the proper function of several metalloenzymes such as arginase, glutamine synthetase, phosphoenolpyruvate decarboxylase and manganese superoxide dismutase.
CONCLUSIONS
Among the studied genotypes were identified genotypes L-047 with 216.3, followed by genotypes LCPM35 and LC027016, with 159.5 and 127.3 mg.kg-1 with the highest iron content, which was higher than the commercial witnesses Caupicor 50 and ICA Betancí.
The contents of zinc, copper, and manganese in caupi bean seeds were similar to those contained in commercial witnesses.
Acknowledgments
The authors thank the University de Córdoba-Colombia, for research funding and soil laboratory of Facultad de Ciencias Agricolas There are genetic differences between the genotypes evaluated, in relation to the content of protein, iron, zinc, copper and manganese.
Due to the genetic variability, genotypes LC-041-016 and LC021016 were identified with29.2 and 29.1%, with the highest protein content, being superior to the commercial witnesses Caupicor 50 and ICA Betancí that presented contents of 25, 7 and 25.5%.
REFERENCES
Antova, G., Stoilova, T. and Ivanova, M. 2014. Proximate and lipid composition of cowpea (Vigna unguiculata L.) cultivated in Bulgaria. Journal of Food Composition and Analysis. 33: 146–152.
Araméndiz, T.H., Espitia, M. y Sierra, C. 2011. Comportamiento agronómico de líneas promisorias de fríjol caupí Vigna unguiculata l. Walp., en el valle del Sinú. Temas Agrarios. 16 (2): 9 – 17
Aramendiz, H., Cardona, C. and Combatt, E. 2016. Contenido Nutricional de Líneas de Fríjol Caupí (Vigna unguiculata L. Walp.) Seleccionadas de una Población Criolla. Información Tecnológica. 27 (2): 53-60.
Aguado, S.G. A., Moreno, G. B., Jiménez, F.B., García, M.E. y R. Preciado,O.R.E. 2012. Impacto de los sideróforos microbianos y fitosidéforos en la asimilación de hierro por las plantas: una síntesis. Revista fitotecnia mexicana. 35(1): 9-21.
Astudillo C. y Blair, M.W. 2008. Contenido de hierro y zinc en la semilla y su respuesta al nivel de fertilización con fósforo en 40 variedades de frijol colombianas. Agronomía Colombiana. 26:471-476.
Black, R.E., Victora, C.G. and Walker, S.P. 2013. Maternal and child undernutrition and overweight in low-income and middle- income countries. Lancet. 382: 427–451.
Bouis, H., Chassy, B. and Ochanda, J. 2003. Genetically modified food crops and their contribution to human nutrition and food quality Trends in Food Science & Technology. Food Science and Technolo-gy.14:191-209.
Belane, A.K. and Dakora, F. D. 2011. Levels of nutritionally important trace elements and macronutrients in edible leaves and grain of 27 nodulated cowpea (Vigna unguiculata L. Walp.) genotypes grown in the Upper West Region of Ghana. Food Chemistry. 125(1): 99-105.
Bost, M., Houdart, S., Oberli, M., Kalonji, E., Huneau, J.F. and Margaritis,I. 2016. Dietary copper and human health: Current evidence and unresolved issues. J Trace Elem Med Biol. 35: 107–115.
Carvalho, A.F., Mateus de Sousa M., Farias, D.F., Rocha-Bezerr, L., Pereira da Silva, R. and Viana, M. 2012. Nutritional ranking of 30 Brazilian genotypes of cowpeas including determination of antioxidant capacity and vitamins. Journal of Food Composition and Analysis. 26 (1):81–88.
DNP. 2014. Dialogo Regional para la Construción del Plan Nacional de Desarrollo 2014-2018. Caribe, Atlantico. Recuperado de. Bases Plan Nacional de Desarrollo: https://colaboracion.dnp.gov.co/cdt/prensa/bases%20plan%20nacion- al%20de%20desarrollo%202014-2018.pdf
De Paula, C., Jarma, O.A. y Aramendiz, H. 2018. Caracterización nutricional y determinación de ácido fítico como factor antinutricional de frijol caupí. Agron. Mesoam. 29(1): 29-40.
Duc, G., Bao, S., Baum, M., Redden, B., Sadiki, M., Suso M.J., Vishniakova, M. and Zong, X. 2010. Diversity maintenance and use of Vicia faba L. genetic resources. Field Crops Res. 115: 270–278.
Espinoza, G.N., Martínez, R. M.R., Chávez-Servia, J.L., Vera-Guzmán, A.M., Carrillo, R.J.C., E. Heredia, G.E y Velasco, V.V.A. 2016. Contenido de minerales en semilla de poblaciones nativas de frijol común (Phaseolus vulgaris L.). Rev. Fitotec. Mex. 39 (3): 215 – 223.
FAO (Organización de las Naciones Unidas para la Alimentación y la Agricultura). 2013. Producción de cultivos. http://faostat.fao.org
Feil, S.B., Moser, S., Jampatong, S. and Stamp, P. 2005. Mineral composition of the grains of tropical maize varieties as affected by pre-anthesis drought and rate of nitrogen fertilization, Crop Sci. 45: 516–523.
Ferguson, B.J., Indrasumunar, A., Hayashi, S., Lin, M.H., Lin, Y.H., Reid, D.E. And Gresshoff, P.M. 2010. Molecular analysis of legume nodule development and autoregulation. J Integr Plant Biol. 52: 61– 76
Frota, K., Soares, R. e Areas, J. 2008. Composição química do feijão caupi (Vigna unguiculata L. Walp), cultivar BRS-Milênio. Ciênc. Tecnol. Aliment. 2 (28):470-476.
Graham, R., Welch, R., Saunders, D., Ortiz-Monasterio, I., Bouis H. and Bonierbale, M. 2007. Nutritious Subsistence Food Sys- tems. Advances in Agronomy, 1-74p.
Gupta, P., Singh, R., Malhotra, S., Boora, K. and Singal, H. 2010. Characterization of seed storage proteins in high protein genotypes of cowpea [Vigna unguiculata (L.) Walp.], Physiol. Mol. Biol. Plants. 53- 57p.
IGAC (Instituto Geográfico Agustín Codazzi). 2006. Métodos analíticos del laboratorio de suelos. Subdirección de Agrología, 6a Edición, Bogotá, Colombia. 499p.
Kassebaum, N.J., Jasrasaria, R. and Naghavi, M. 2014. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 123: 615–624.
Kouchi, H., Imaizumi-Anraku, H., Hayashi, M., Hakoyama, T., Nakagawa, T., Umehara., Y., Suganuma, N. and Kawaguchi, M. 2010. How many peas are in a pod? Legume genes are responsible for mutualistic symbioses underground. Plant Cell Physiol. 51: 1381–1397
Li, L., and Yang, X. 2018. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid Med Cell Longev. Article ID 7580707, 11 pages. Doi: 10.1155/2018/7580707
Masuda, H.M., Suzuki, M., Kobayashi, T., Nakanishi, N., Takahashi, M. and Saigusa M. 2008. Increase in Iron and Zinc Concentrations in Rice Grains Via the Introduction of Barley Genes Involved in Phytosiderophore Synthesis. Rice. 2: 155-166.
Magulu, K. and Kabambe, V. 2015. Fodder production, yield and nodulation of some elite cowpea (Vigna unguiculata [L.] Walp.) lines in central Malawi. Afr J Agric Res. 10(25):2480- 2485.
Martinez, O. y Martinez, E. 2006. Proteínas y péptidos en nutrición enteral. Nutr. Hosp. 21(2): 1-14.
Mesquita, F., Corrêa, A., Patto de Abreu, C., Zambaldi, R. A. and De Fátima Barbosa, A. 2007. Linhagens de feijão (Phaseolus vulgaris L.): composição química e digestibilidade protéica. Ciênc. agrotec., Lavras. 31(4): 1114-1121.
Nair, R.M., Thavarajah, D., Thavarajah, P., Giri, R., Ledesma, D. and Yang, R. 2015. Mineral and phenolic concentrations of mungbean [Vigna radiata (L.) R. Wilczek var. radiata] grown in semi-arid tropical India. Journal of Food Composition and Analysis.39 (1): 23–32.
Naz, N., Hameed, M., Nawaz, T., Aqeel Ahmad M. S. and Ashraf, M. 2013. Soil-plant relationships in the arid saline desert of Cholistan. Arid Land Research and Management. 7: 140–152.
Nestel, P., Bouis, H., Meenakshi, J. and Wolfgang, P. 2006. Biofortification of staple food crops. The journal of Nutrition. 136: 1064-1067.
Palencia, G., Mercado, T. y Combatt, E. 2006. Estudio agroclimático del departamento de Córdoba, Ed. Gráficas el Caribe, Montería, Colombia, 126p.
Ramos de Vega, M. and Sangronis. E. 2006. Influencia de la germinación en la composición del Phaseolus vulgaris y Vigna sinensis. Agronomía Tropical. 56(4): 531-537.
Reilly, C. 2004.The Nutritional Trace Metals. Brisbane, Australia: Blackwell Publishing Ltd, 238p.
Sadras, V.O., Rebetzke, G.J. and Edmeades, G.O. 2013. The phenotype and the components of phenotypic variance of crop traits. Field Crop Res. 154: 255–259.
Saletta, F., Rahmanto, Y. S., Noulsri, E. and Richardson, D. R. 2010. Iron chelator-mediated alterations in gene expression: identification of novel iron- regulated molecules that are molecular targets of hypoxia-inducible factor-1α and p53. Molecular Pharmacology, 77(3), 443-458.
Salgado, S. M., Guerra, N.B., Andrade, S. A. C. and Livera A. V. S. 2005. Caracterização físico-química do grânulo do amido de feijão-caupi. Ciência e Tecnologia de Alimentos. 25(3): 525-530.
Scott, S.P., Chen-Edinboro, L.P. and Caulfield, L.E. 2014. The impact of anemia on child mortality: an updated review. Nutrients. 6:5915–5932.
Stein, A.J. 2010. Global impacts of human malnutrition. Plant Soil.335: 133–154.
Tolga, K., Ahmet, D., Faruk, T., Gürsoy, N., Tugay., E. K., Uncuer, D. and Özkan, H. 2018. Assessment of micro and macronutrient contents in the Turkish faba bean germplasm. 1: 72–78.
Vargas, Y. R., Villamil, O. E., Murillo, E., Murillo, W. y Solanilla, J. F. 2012. Caracterización fisicoquímica y nutricional de la harina de frijol caupí Vigna unguiculata L. cultivado en Colombia. Vitae. 19(1): 320- 321.
White, P. y Broadley, M. 2016. Biofortification of crops with seven mineral elements often lacking in human diets iron, zinc, copper, calcium, magnesium, selenium and iodine https://doi.org/10.1111/j.1469-8137.2008.02738
Notes

